Research
Overall goal and motivation
After injury, axons in the adult mammalian central nervous system fail to regenerate, which is a major contributor to sustained motor, sensory, and autonomic dysfunction following spinal cord injury. Our goals are to understand the cellular and molecular mechanisms that prevent regeneration and to develop an effective therapy that restores function following spinal cord injury.
Approach
|
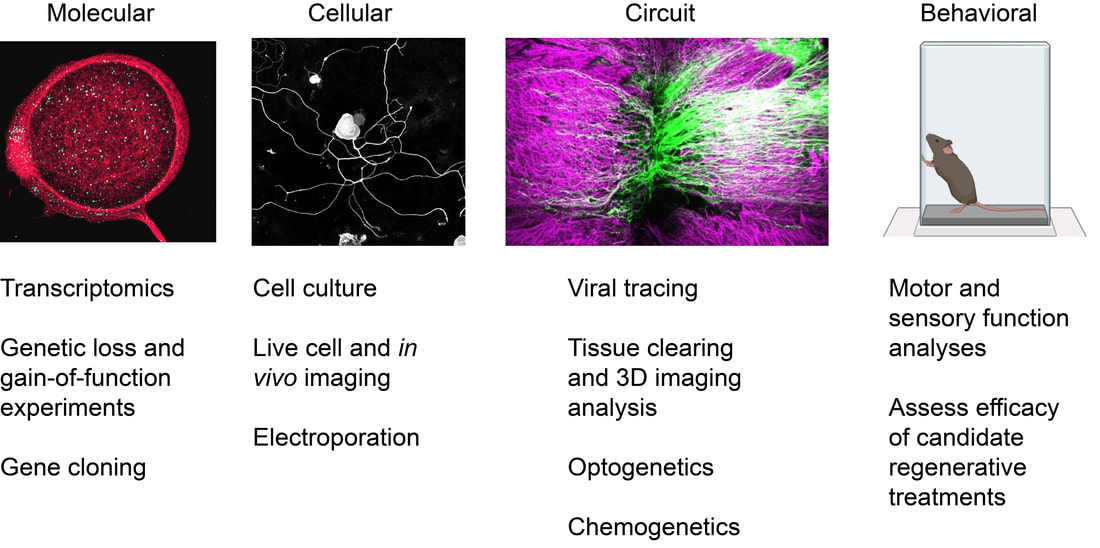
We use a multidisciplinary approach combining cellular, molecular, and bioinformatic approaches together with cutting-edge microscopy and behavioural analyses. We aim to understand the biology of axon growth, how newly grown axons can be coaxed into forming functional circuits, and how experimental strategies that enhance regeneration can be combined in a rational way to restore lost function.
|
Projects
|
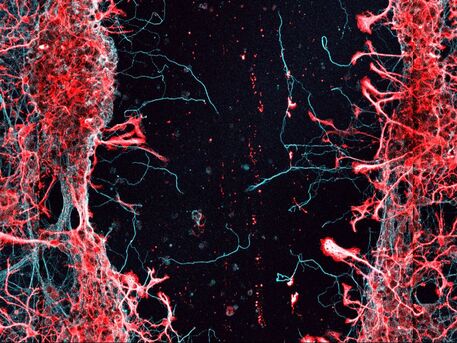
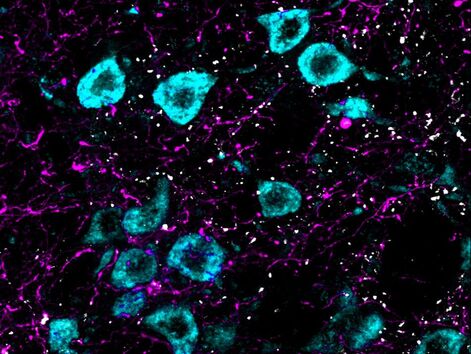
Cellular and molecular mechanisms of axon growth. Central mammalian neurons lose their capacity to effectively regenerate axons during embryonic or early postnatal development. We are interested in understanding how neuronal maturation restricts regeneration with a particular focus on the role of the synapse and synapse-related molecules. In addition, we investigate cell-cell interactions between neurons and other cell types (astrocytes, oligodendrocyte precursor cells) that can enable or restrict regeneration.
|
|
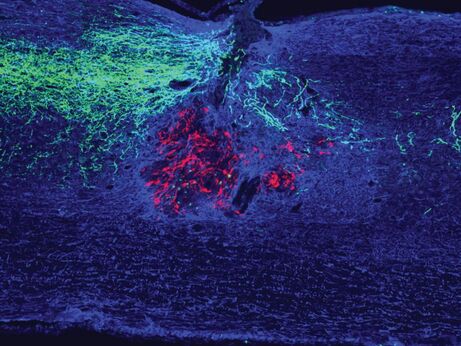
Experimental strategies to coax regeneration and functional improvements following spinal cord injury. Despite decades of progress, there is still no regenerative therapy that can reverse paralysis and other dysfunctions following spinal cord injury. We aim to develop an effective and clinically feasible regenerative treatment for the injured spinal cord. We combine therapies that augment the intracellular capacity of the neuron for growth with lesion site-directed therapies such as cellular transplantation to maximize the extent of regeneration that can occur, then vigorously test these combinations for their capacity to enable regeneration and functional improvements.
|
|
Connectomics of spinal cord injury. Although spinal cord injury often results in severe and permanent motor, sensory, and autonomic dysfunction, spontaneous functional improvements can and do occur. However, the most relevant pathways and cell types that mediate these improvements are unclear. We are applying tissue clearing and 3D imaging analysis together with optogenetic and chemogenetic strategies to better understand the most relevant cells and circuits in mediating functional improvements following injury. Through this, we hope to develop strategies to target these circuits in a way that enables functional improvements. |